This is why success requires many shots on goal says Thomas noting there have been more than 200 COVID-19 vaccines in development. What will it take for FDA to award full approval for vaccine.
 Opinion How Long Will A Vaccine Really Take The New York Times
Opinion How Long Will A Vaccine Really Take The New York Times
By Jessica Dyer February 3 2021 at 959 PM EST - Updated February 3 at 959 PM CHARLOTTE NC.
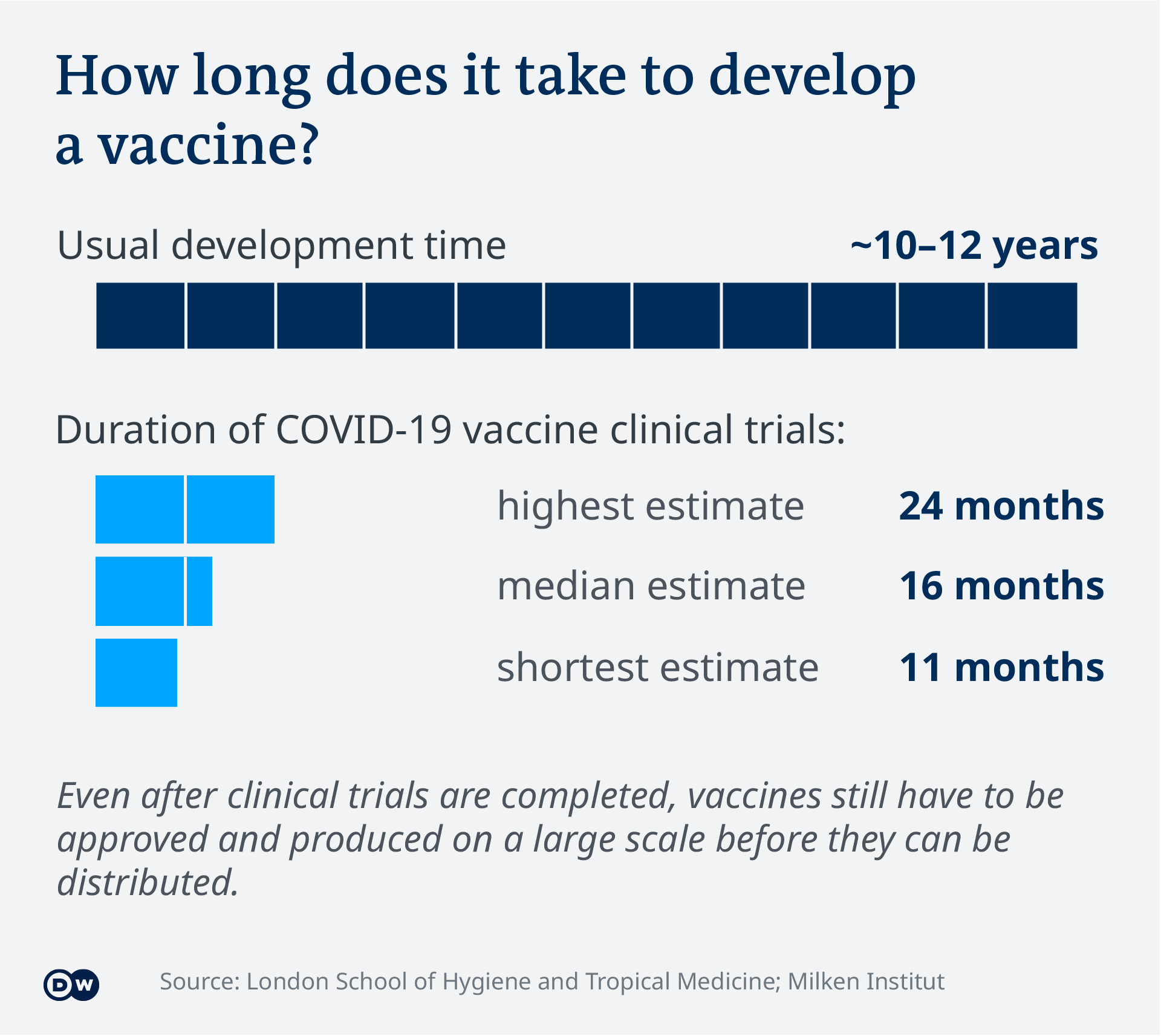
How long does it take to approve a vaccine. The FDA works to ensure that all new vaccines on the market are safe effective and have minimal side. The MenB Meningitis B vaccine took nearly 20 years from the first idea to the vaccine. In total a vaccine can take more than 10 years to fully develop and costs up to 500 million the UK charity says.
The process of getting a vaccine approved for use in the general public is no picnic and can take several years. How long do I have to wait before getting other vaccines. No matter what only a safe effective vaccine will get our approval.
There is no data on administering COVID-19 vaccine at the same time as other non-COVID-19 vaccines. The circumstances have changed considerably since Dec. But the FDA took only about 2 12 weeks to review the Moderna data.
Thats the same standard the FDA has had for years Dr. For other vaccines phase 2. It can take years.
What is the usual process for developing a vaccine. WBTV - There doesnt seem to be a lot of conversation about the fact that the FDA has only provided emergency authorization for current vaccines. It usually takes 10 to 105 years to develop a vaccine which makes the existing COVID-19 vaccines on the market all the more incredible.
In the United States vaccines are regulated by the Food and Drug Administrations Center for Biologics Evaluation and Research. COVID-19 and the FDA. The discovery and research phase is normally two-to-five years according to the Wellcome Trust.
To get full approval known as a Biologics License companies will need to submit six months of data. From a safety perspective FDA expects an EUA submission will include all safety data accumulated from phase 1 and 2 studies conducted with the vaccine with an expectation that phase 3 data will. If we are talking about a vaccine that has already been tested and approved we could generalize and say that one batch of vaccine consisting of a couple thousand doses may take.
Its not unusual for a vaccine to take 10 to 15 years to complete all the phases under normal circumstances. How long does it take the MHRA to approve vaccines. Wolfe said one he thinks will make a.
Traditionally vaccine development takes several years and includes various processes including design and development stages followed by. Usually scientists conduct several phase 2 studies involving a few hundred patients per trial. Its discovered that flu viruses change from year to year and the vaccine will need to be adjusted annually to be effective.
These stages are mandated by the Food and Drug Administration FDA with its Center for Biologics Evaluation and Research CBER division officially in charge of regulating vaccines. Why three weeks for JJ. Phase 2 trials for COVID-19 vaccines are expected to take eight months.
Or 14 days or less. Other vaccines should not be administered 14 days or less before the first dose. If we are talking about a vaccine that has already been tested and approved we could generalize and say that one batch of vaccine consisting of a couple thousand doses may take 2-6 weeks to go from starting with raw materials to being a completed vaccine in a vial or syringe.
A minimum interval of 14 days is recommended before or after any other vaccines ie. Dr Hahn discusses the agencys role in responding to the COVID. The first flu vaccine is licensed for civilian use in the US-1947.
 Opinion How Long Will A Vaccine Really Take The New York Times
Opinion How Long Will A Vaccine Really Take The New York Times
 How Long Does It Take To Develop A Vaccine World Economic Forum
How Long Does It Take To Develop A Vaccine World Economic Forum
 Opinion How Long Will A Vaccine Really Take The New York Times
Opinion How Long Will A Vaccine Really Take The New York Times
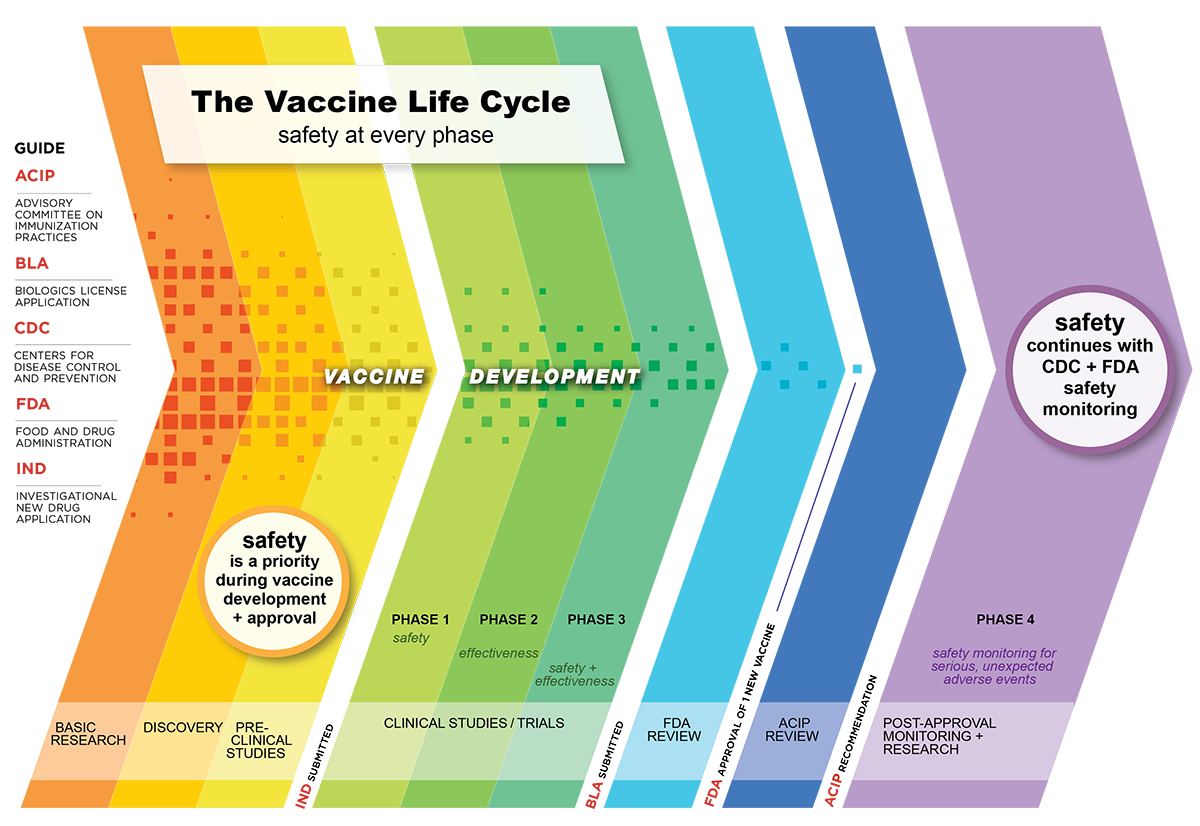 U S Vaccine Safety Overview History And How It Works Cdc
U S Vaccine Safety Overview History And How It Works Cdc
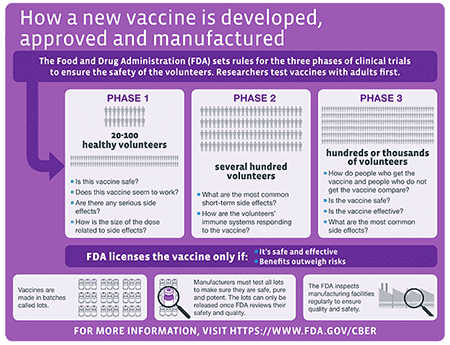 Ensuring The Safety Of Vaccines In The United States Cdc
Ensuring The Safety Of Vaccines In The United States Cdc
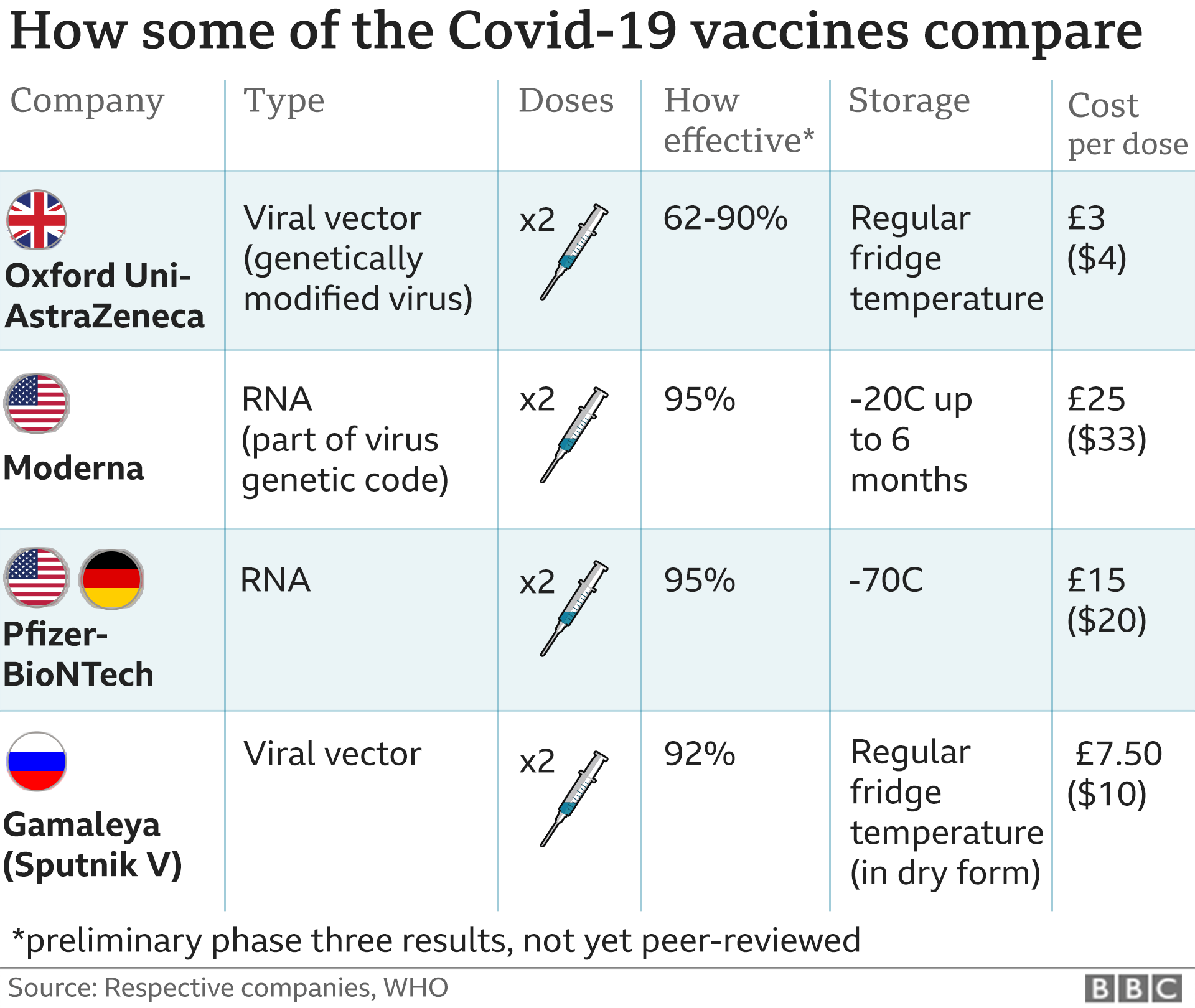 Covid Vaccine Moderna Seeks Approval In Us And Europe Bbc News
Covid Vaccine Moderna Seeks Approval In Us And Europe Bbc News
 Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
 Covid 19 Vaccines Development Evaluation Approval And Monitoring European Medicines Agency
Covid 19 Vaccines Development Evaluation Approval And Monitoring European Medicines Agency

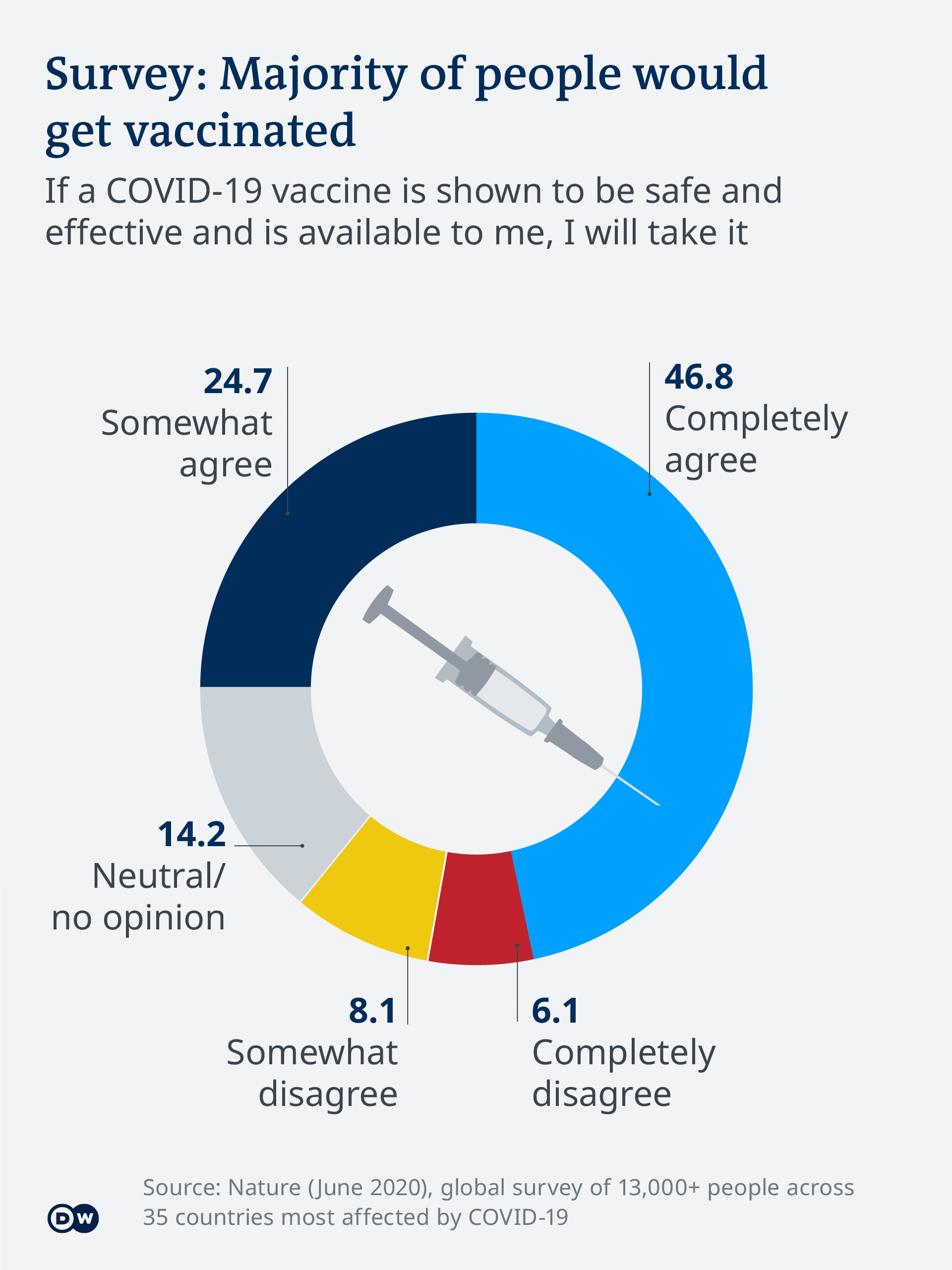 Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
Covid 19 Vaccine Development What S The Progress Science In Depth Reporting On Science And Technology Dw 16 04 2021
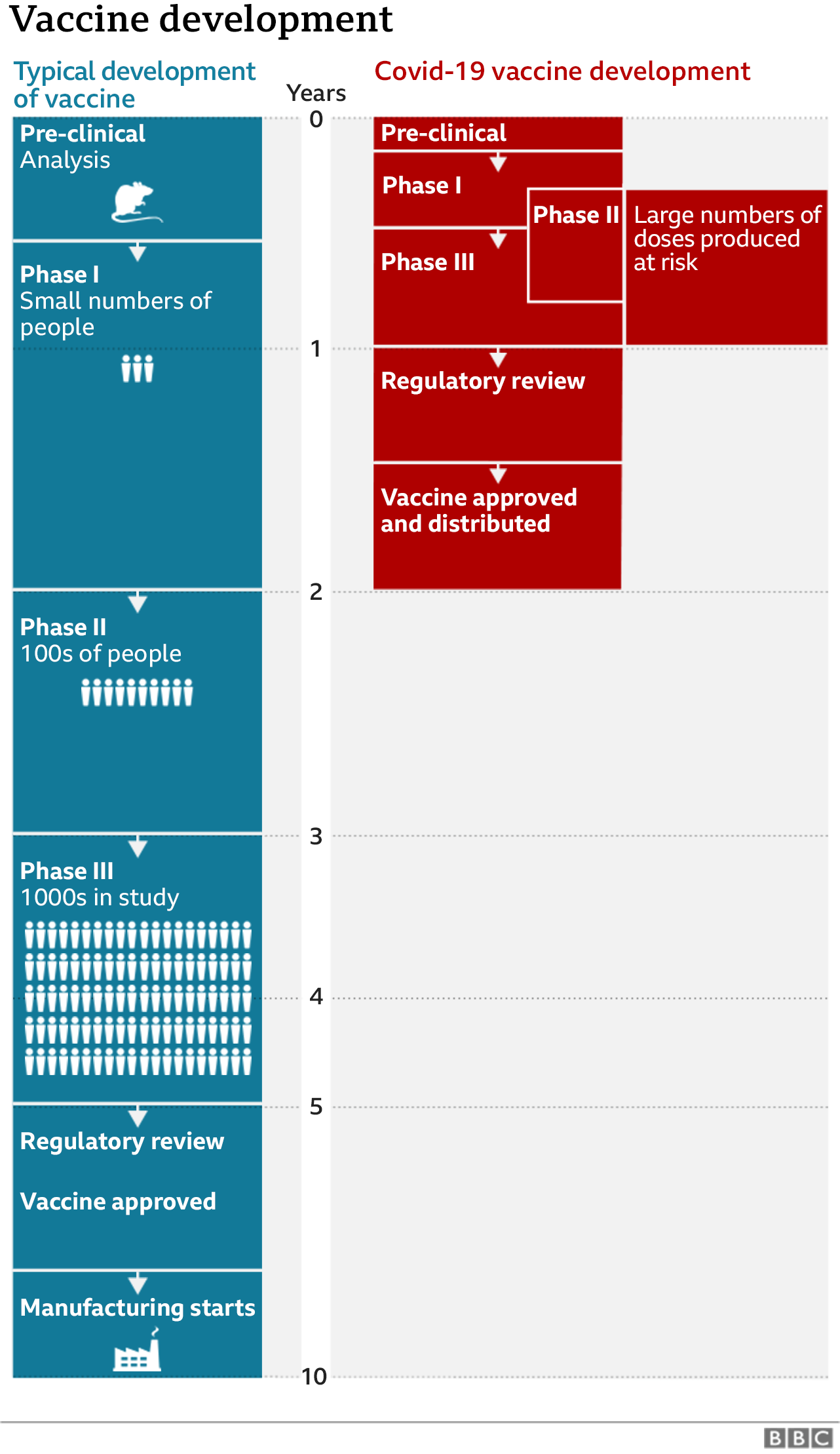 Coronavirus How Soon Can We Expect A Working Vaccine Bbc News
Coronavirus How Soon Can We Expect A Working Vaccine Bbc News
 Here S What Needs To Happen Before We Can All Get Vaccinated For Covid 19 Cbc News
Here S What Needs To Happen Before We Can All Get Vaccinated For Covid 19 Cbc News
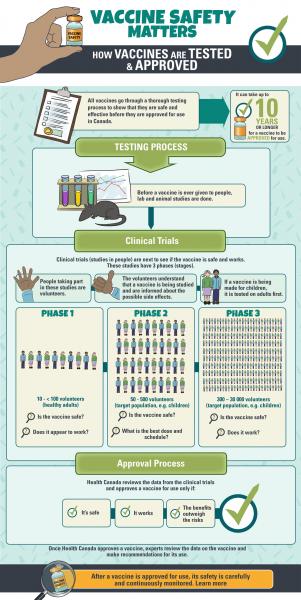 Testing Approval And Monitoring Immunize Bc
Testing Approval And Monitoring Immunize Bc

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.